Business Scope

In-vitro diagnosis and bio-healthcare services
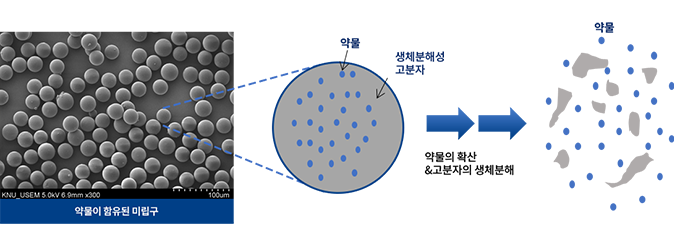
We’re developing innovative products and services focusing on the prevention and diagnosis of diseases, and monitoring patients using various types and forms of nanoparticles such as Graphene, Carbon Nanotube, Magnetic Beads, etc.

Femtech Supplies for women
We have been R&D and newly commercializing Femtech Products for women life better, along with varied shape of feminine cleansers & wipes and medical devices as pregnancy testers, ovulation testers, vaginitis test kit and urethritis test kit etc.
Technology Development






Main products






Company information
10-5, Myeonghaksandanseo-ro, Yeondong-myeon, Sejong Special Self-Governing City
+82-44-862-9134
Fax+82-44-862-9135