01.
3rd line treatment for Stomach cancer
A global phase 3 clinical trial has been completed for Rivoceranib as a monotherapy for the third-line treatment of stomach cancer.
Rivoceranib is an oral targeted anticancer agent designed to selectively inhibit vascular endothelial growth factor receptor 2 (VEGFR-2), a key player in the formation of new blood vessels within tumors.
It is the world's first synthetic VEGFR-2 inhibitor drug approved as a treatment for stomach cancer, and is currently available on the market in China.
Furthermore, clinical trials involving thousands of patients across multiple countries, including the United States, Europe, Korea, and Japan, are underway to validate Rivoceranib's efficacy across various cancer types, including stomach cancer, colon cancer, blood cancer, non-small cell lung cancer, esophageal cancer, and thyroid cancer.
Rivoceranib has demonstrated significantly enhanced results in combination and maintenance therapies when paired with various medications, including chemotherapy and immunotherapy. Currently, it is undergoing various global clinical trials, including indication expansion studies and combination studies.
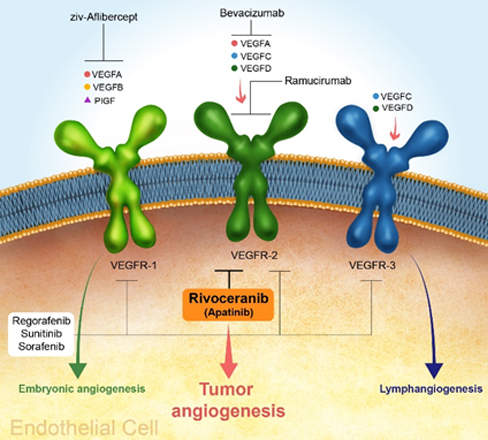
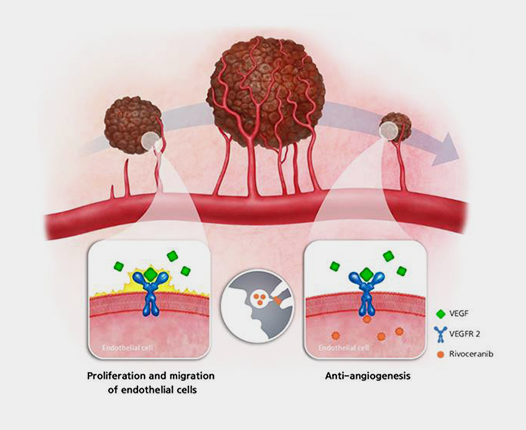
Small tumors can receive oxygen supply through diffusion and can survive without blood vessels, but in order to grow to a diameter of 2 mm or more, the supply of oxygen and nutrients through blood is essential. Cancer cells secrete vascular endothelial growth factor (VEGF) for growth, and when VEGF binds to receptors on the surface of vascular endothelial cells, it triggers angiogenesis, facilitating the delivery of oxygen and nutrients to the tumor. Additionally, metastasis may occur through new blood vessels.
VEGF, a key promoter of angiogenesis, includes VEGF-A, VEGF-B, VEGF-C, VEGF-D, and PIGF (placental growth factor). Vascular endothelial cells contain vascular endothelial cell receptors (VEGFR)-1, 2, and 3 that accept vascular endothelial growth factor, and among these, VEGFR-2 is involved in the formation of new blood vessels in cancer.
Rivoceranib is a small molecule Tyrosine Kinase Inhibitor (TKI) that selectively targets VEGFR-2, a key player in the formation of new blood vessels in cancer. By blocking the signaling pathway of vascular endothelial cells, Rivoceranib effectively prevents the formation of new blood vessels by inhibiting cell proliferation and migration.
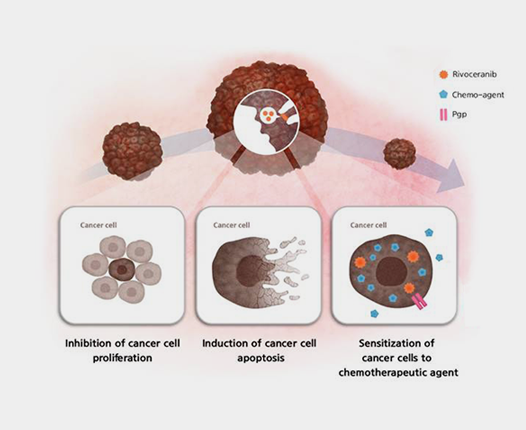
Two important cellular processes in our body are proliferation and apoptosis. However, unlike normal cells, cancer cells continuously proliferate without controlling the proliferation and death process. Rivoceranib inhibits the proliferation of cancer cells and induces cancer cell death, by inhibiting the proliferation of cancer cells and inducing their death. By doing so, it prevents tumor progression in various types of cancer, including liver cancer, colon cancer, ovarian cancer, and acute leukemia. Studies have shown that it inhibits tumor growth in various cancers.
The leading cause of failure in anticancer drug treatment is multidrug resistance, wherein cancer cells develop resistance to multiple types of anticancer drugs simultaneously. This resistance often is caused by the overproduction of proteins like P-glycoprotein (P-gp) or multidrug resistance-related proteins in the cell membrane of cancer cells. These proteins block the entry of anticancer drugs into the cells while increasing the expulsion of drugs that have already entered, thereby diminishing the effectiveness of the treatment. Furthermore, they can change the structure of the anticancer drugs inside the cell, rendering them ineffective.
by inhibiting the activity of P-glycoprotein, thereby maintaining adequate concentrations of anticancer drugs within the cells , which increases multidrug resistance or drug release.
In this way, Rivoceranib can not only increase the effectiveness of other anticancer drugs, but is also effective in tumors that are resistant to anticancer drugs, making it a valuable addition in combination therapy.
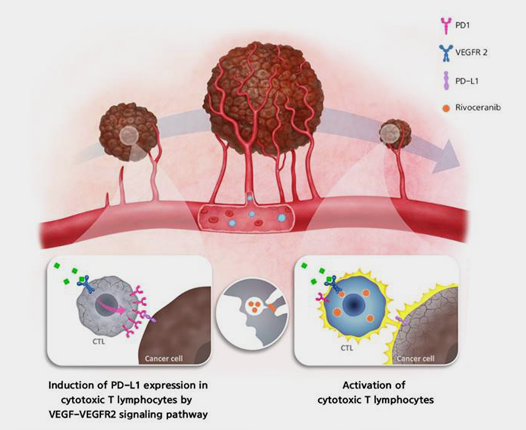
VEGF/VEGFR2 plays a significant role not only in blood vessel formation but also in modulating immunity within the tumor microenvironment. It promotes the induction and proliferation of Regulatory T cells (Tregs) and Myeloid-Derived Suppressor Cells (MDSCs), which exert immunosuppressive effects. Conversely, it can inhibit the activity of Cytotoxic T lympnocytes (CTLs) and dendritic cells (DCs), which are crucial for activating immune responses. In particular, the weakening of CTLs is known to be due to increased expression of immune checkpoints such as PD-1, CTLA-4, Tim-3, and Lag-3 through the VEGFR2 signaling pathway.
Rivoceranib is known to decrease the expression of immune checkpoints such as PD-1, Tim-3, and Lag-3 in cytotoxic T cells (CTLs) while also enhancing the secretion of immune-activating factors like IFN-r and IL-2. This activates immunity within the tumor microenvironment. Consequently, Rivoceranib is anticipated to exert a synergistic effect when used in combination therapy with immune checkpoint inhibitors.
The stomach, the largest digestive organ in our body, is situated in the upper left part of the abdomen, positioned beneath the ribs and within the solar plexus. It is a sac-shaped organ that connects to the esophagus above and the duodenum below.
Functionally, the stomach serves as both a reservoir for food storage and a site for digestion to occur.
Stomach cancer broadly encompasses all cancers that develop within the stomach, but it predominantly refers to gastric adenocarcinoma, originating from the glandular cells of the gastric mucosa. Adenocarcinoma is further categorized into various types based on the observed shape of cancer cells under a microscope. In addition to gastric adenocarcinoma, rare types of stomach cancer include lymphoma, arising from lymphoid tissue, gastrointestinal stromal tumor, originating from gastric stromal cells, sarcoma, a malignant tumor derived from non-epithelial cells, and neuroendocrine tumors that secrete hormones. Neuroendocrine tumors, also known as carcinoid tumors, can also occur in the stomach.
The colon, or large intestine, is a long, tube-shaped digestive organ that begins at the end of the small intestine and connects to the anus. It is about 150 cm long. Although it is much shorter than the small intestine, which typically exceeds 6 meters, the colon is wider, hence the name "large intestine." Cancer that occurs in this area is known as colon cancer.
The large intestine is anatomically divided into several sections: the appendix, cecum, colon, rectum, and anal canal. The colon, in turn, is further subdivided into the ascending colon, transverse colon, descending colon, and sigmoid colon. Malignant tumors that develop in the appendix, colon, and rectum are collectively referred to as colon cancer. The most common type of colon cancer is adenocarcinoma, which originates in the glandular cells of the mucous membrane. However, other primary forms of cancer can also occur in the large intestine, including lymphomas, malignant carcinoids, and leiomyosarcomas.
The liver is the largest organ in our body, located just below the diaphragm and inside the ribs beneath the right breast when viewed from the outside.
Liver cancer refers to a primary malignant tumor that originates in the liver. Although the general public often refers to cancer that has spread to the liver from other organs as liver cancer, the term strictly applies only to primary liver cancer. Pathologically, there are various types of primary liver cancer, including hepatocellular carcinoma, cholangiocarcinoma, hepatoblastoma, and hemangiosarcoma, with hepatocellular carcinoma and cholangiocarcinoma being the most common.
Adenoid cystic carcinoma (ACC) is a rare cancer that primarily occurs in the salivary glands but can also develop in the breasts, skin, respiratory tract, and reproductive organs. ACC is a malignant tumor known for its high metastatic potential and tendency to develop around nerves. Although its growth rate is slow, the cure rate remains low due to its high recurrence rate, which is approximately 40% or more after initial treatment, and its aggressive nature. If recurrence or metastasis occurs following surgery or radiation therapy, chemotherapy becomes necessary.
Combination chemotherapy regimens, such as doxorubicin, cisplatin, and cyclophosphamide, are being used, but their effectiveness is unclear, and there is no standard anticancer treatment for ACC.
* Source: National Cancer Information Center

A global phase 3 clinical trial has been completed for Rivoceranib as a monotherapy for the third-line treatment of stomach cancer.
A global phase 3 clinical trial has been completed for the combination therapy of Rivoceranib and Camrelizumab (a PD-1 antibody therapeutic) developed by China's Hengshe Pharmaceutical for the first-line treatment of liver cancer.
Phase 2 clinical trials of Rivoceranib monotherapy for the first-line treatment of adenoid cystic carcinoma have been completed in the United States and Korea.
Phase 1/2 clinical trials are underway in Korea for the combination therapy of Rivoceranib and Paclitaxel for the second-line treatment of gastric cancer.
Phase 1/2 clinical trials are underway in the U.S. and Korea for the combination therapy of Rivoceranib and Lonsurf, developed by Japan's Taiho Pharmaceutical, for the third-line treatment of colon cancer.
A phase 1 trial of the combination therapy of Rivoceranib and Opdivo, developed by global pharmaceutical company BMS, for sarcoma and various solid tumors is underway in the United States.